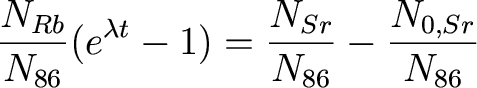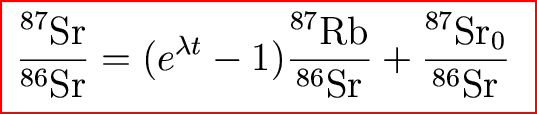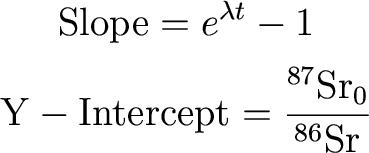Radioactive Age Dating, Part 2
Okay, so last time we were examining the decay of radioactive
isotopes, for example:
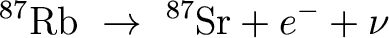 Here, 87Rb is referred to as the parent
isotope and 87Sr is referred to as the daughter
isotope.
Here, 87Rb is referred to as the parent
isotope and 87Sr is referred to as the daughter
isotope.
And we found that the decay rate went like this:
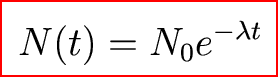 But we don't know the initial abundances of the isotope.
So how can we age date?
But we don't know the initial abundances of the isotope.
So how can we age date?
We do know another piece of information: the
total amount of 87Rb + 87Sr stays constant!
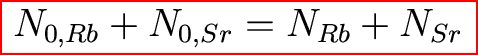 So, time for some algebra. We had
So, time for some algebra. We had
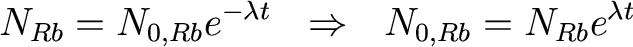
 Plugging and chugging,
we get
Plugging and chugging,
we get
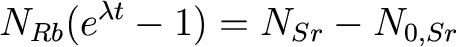 In practice, we examine isotopic ratios, comparing
to a stable isotope, for example (86)Sr.
In practice, we examine isotopic ratios, comparing
to a stable isotope, for example (86)Sr.
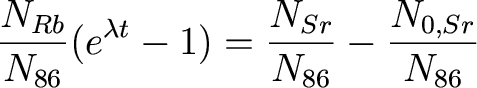 Now, we just rearrange terms (and redo the notation a bit)
to get
Now, we just rearrange terms (and redo the notation a bit)
to get
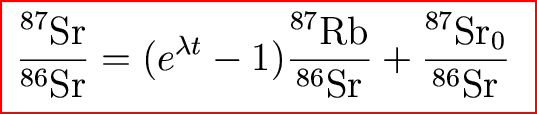 We still have two unknowns
in this equation: t (time) and (87)Sr_0/(86)Sr
(the initial abundance ratio of the Sr isotopes). What now?
We still have two unknowns
in this equation: t (time) and (87)Sr_0/(86)Sr
(the initial abundance ratio of the Sr isotopes). What now?
What does that last equation look like? A
line! So:
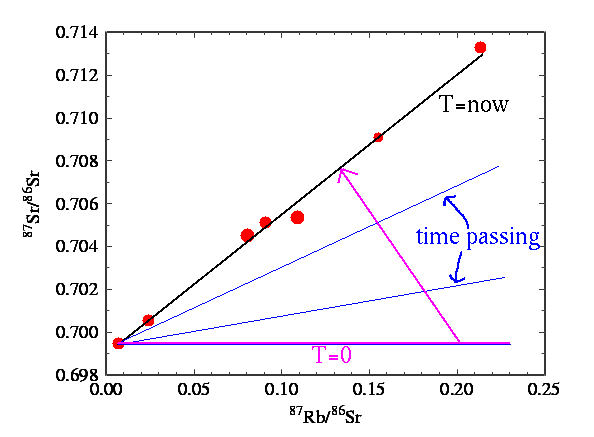
-
Measure the abundance
ratios at several places in the rock
-
Plot daughter isotope
vs parent isotope.
-
Fit a straight line to
the data. Then
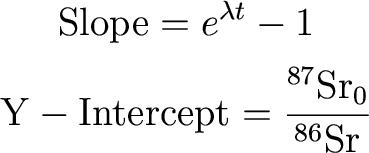 So we can solve for t and the initial Sr isotopic abundance
ratios. Cool!
So we can solve for t and the initial Sr isotopic abundance
ratios. Cool!
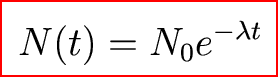
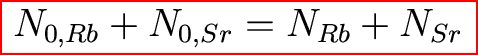
 Plugging and chugging,
we get
Plugging and chugging,
we get